38 phase labels in chemical equations
Structures, Phase Transitions and Tricritical Behavior of the … Oct 21, 2016 · We have examined the crystal structures and structural phase transitions of the deuterated, partially deuterated and hydrogenous organic-inorganic hybrid perovskite methyl ammonium lead iodide ... Phase Definition and Examples - ThoughtCo In chemistry and physics, a phase is a physically distinctive form of matter, such as a solid, liquid, gas, or plasma. A phase of matter is characterized by having relatively uniform chemical and physical properties. Phases are different from states of matter.
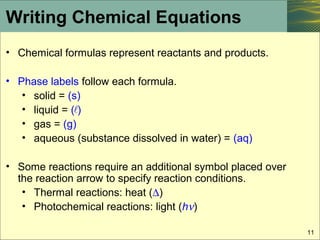
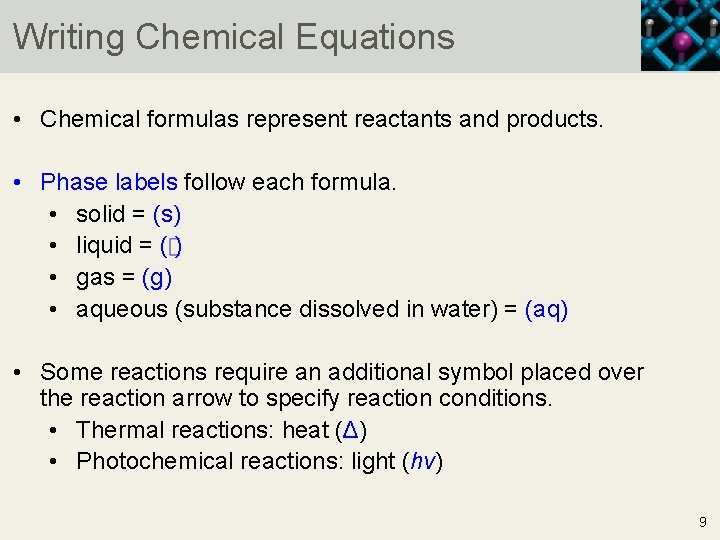
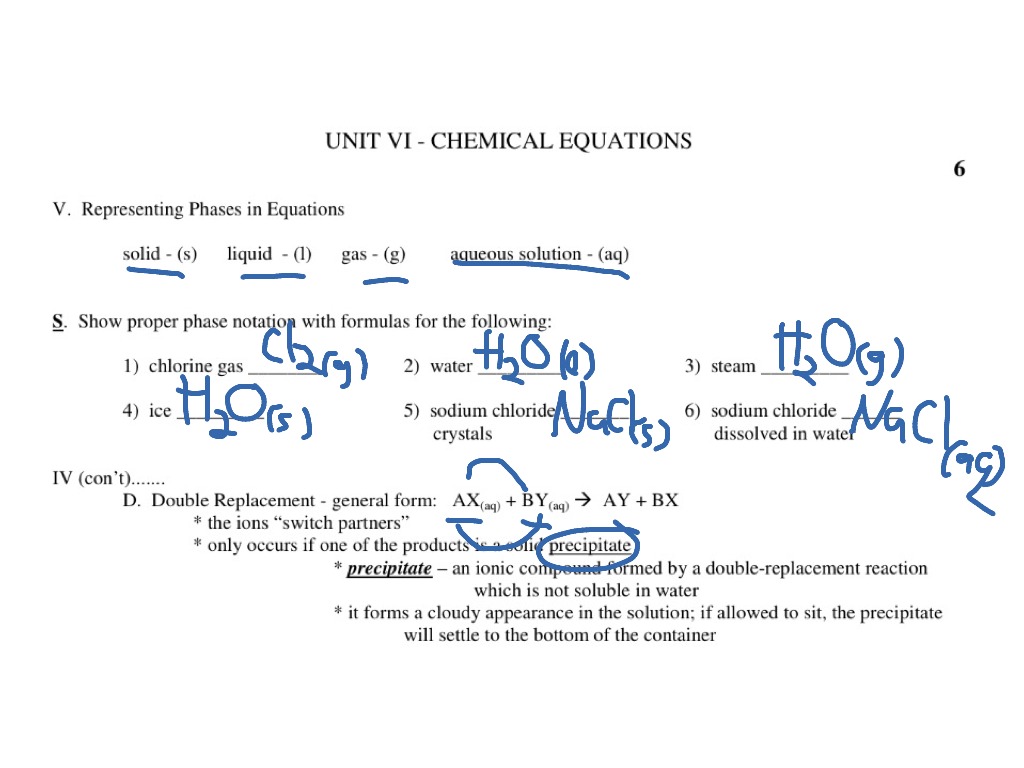
The Chemical Equation - GitHub Pages Many chemical equations also include phase labels for the substances: (s) for solid, (ℓ) for liquid, (g) for gas, and (aq) for aqueous (i.e., dissolved in water). Special conditions, such as temperature, may also be listed above the arrow. For example, Key Takeaways A chemical equation is a concise description of a chemical reaction.

Phase labels in chemical equations
Properties of the density functional response kernels and its ... Sep 15, 2022 · When considering the particle-density operator, there are different possibilities, emerging from different possibilities in the labels s and t (and u and v) in the particle-density operator: The labels s and t are associated with the wavefunction of different fragments in the bra and ket, e.g., s with fragment A and t with fragment B , The Chemical Equation - Introductory Chemistry - 1st Canadian Edition Write and balance the chemical equation that represents nitrogen and hydrogen reacting to produce ammonia, NH 3. Answer N 2 + 3H 2 → 2NH 3 Many chemical equations also include phase labels for the substances: (s) for solid, (ℓ) for liquid, (g) for gas, and (aq) for aqueous (i.e., dissolved in water). Definition of dissolve and the state label (aq) in chemical equations A colloid is a heterogeneous phase. But above all, do not worry too much about these minor symbolism issues. A chemical equation is not meant to convey everything. It is just a shorthand notation to describe chemical reactions. A real experiment or a research paper should describe the experimental condition with sufficient details in such a way ...
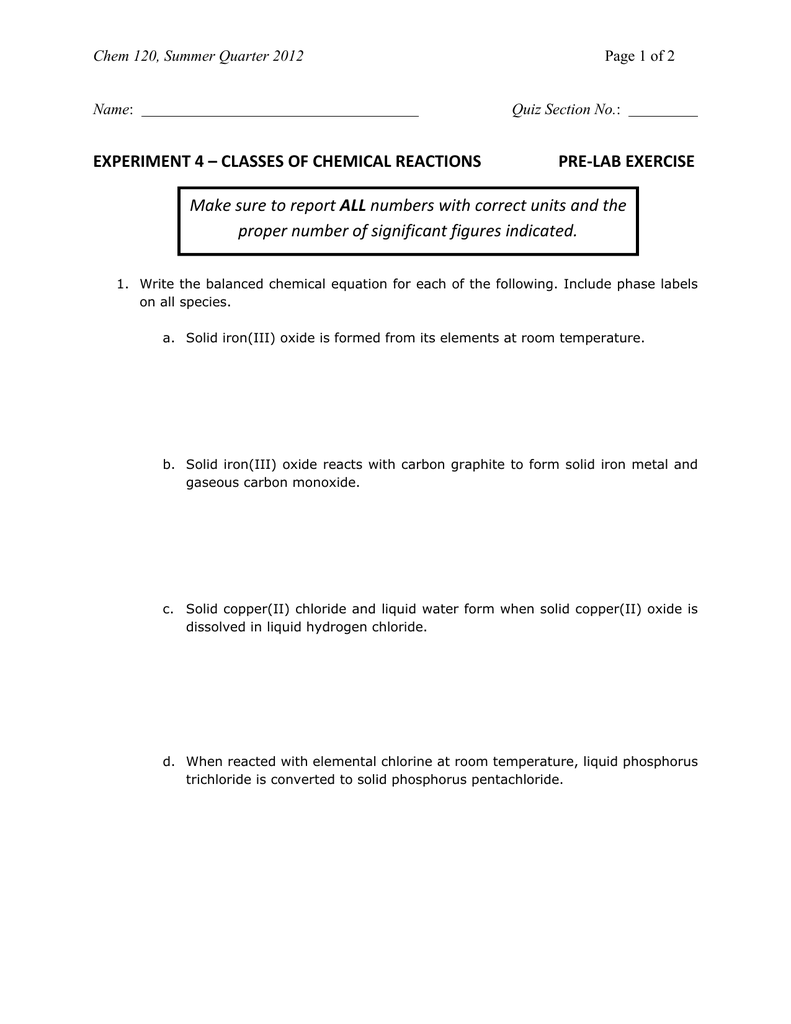
Phase labels in chemical equations. Optical Metasurfaces for Energy Conversion | Chemical Reviews Nanostructured surfaces with designed optical functionalities, such as metasurfaces, allow efficient harvesting of light at the nanoscale, enhancing light–matter interactions for a wide variety of material combinations. Exploiting light-driven matter excitations in these artificial materials opens up a new dimension in the conversion and management of energy at the nanoscale. In this review ... Chapter 7 The chemical equation that can be written to describe the solubility of sodium chloride is, NaCl (s)---H 2 O--> Na + (aq) + Cl-(aq) We can write the equation to describe what happens when copper(II) chloride dissolves in water, ... Write the formula and identify the phase for the product(s) and balance the following reaction. Na 2 SO 4(aq ... PDF Chemical reaction : conversion of substances into different substances ... Phase labels : show the phase of reactants or products (s) : (l) : (g) : (aq ) : Chapter 7: Chemical Reactions ch7a Page 1 ___ H 2+ ___ O 2 → ___ H 2O Law of conservation of mass: 1. Leave elemental substances for last: ... Use the chemical equations worksheet to practice writing and balancing chemical equations. Solved 2. Chemical equations can also be used to represent | Chegg.com Write a chemical reaction for the freezing of water, including the proper phase labels. 3. Explain why 4Na (s) + 2C12 (9) - 4NaCl (s) should not be considered a proper chemical equation. 4. Explain why H2 (g) + 1/2O2 (g) → H20 (1) should not be considered a proper chemical equation. 5. Does Question: 2.
Electrochemcal Impedance Spectroscopy (EIS) Basics 27.09.2022 · The impedance is proportional to the frequency-dependent voltage and frequency-dependent current , where is the angular frequency of an oscillating sine wave. The definition of impedance comes from electrical circuits, and as a result, voltage is commonly used to define impedance. However, in electrochemical impedance spectroscopy, we will switch from using … Solved 1. Write a balanced thermochemical equation with | Chegg.com Question: 1. Write a balanced thermochemical equation with phase labels for the Haber process with the heat energy as part of the equation. (3 pts) 2. What is the theoretical yield of ammonia (in grams) if 16.55 grams of nitrogen gas and 10.15 grams of hydrogen gas are allowed to react? (9 pts) 3. chemical equation | Yeah Chemistry Formulas of the reactants appear on the left side of the equation; those of products are written on the right. In many cases it is useful to indicate the state or phases of the substance in an equation.You use the following phase labels: (g) = gas , (l) = liquid , (s) = solid , (aq) = water solution 6.3 Acid-Base Reactions - CHEM 1114 - Introduction to ... - BCcampus The process represented by this equation confirms that hydrogen chloride is an acid. When dissolved in water, H 3 O + ions are produced by a chemical reaction in which H + ions are transferred from HCl molecules to H 2 O molecules ().. Figure 1. When hydrogen chloride gas dissolves in water, (a) it reacts as an acid, transferring protons to water molecules to yield (b) hydronium ions (and ...
5.7 End-of-Chapter Material - Introductory Chemistry - NSCC Write a chemical reaction for the boiling of water, including the proper phase labels. Chemical equations can also be used to represent physical processes. Write a chemical reaction for the freezing of water, including the proper phase labels. Explain why. 4 Na(s) + 2 Cl 2 (g) → 4 NaCl(s) Answered: Complete and balance the three… | bartleby Phase labels are optional. HNO, (aq) + H,O (1) H,0 Ba (ОН), (8) HF (aq) + H,O (1) = Complete and balance the three equations according to acid and base behavior in water. Phase labels are optional. HNO, (aq) + H,O (1) H,0 Ba (ОН), (8) HF (aq) + H,O (1) = Question Analyzing Learned Molecular Representations for Property ... Advancements in neural machinery have led to a wide range of algorithmic solutions for molecular property prediction. Two classes of models in particular have yielded promising results: neural networks applied to computed molecular fingerprints or expert-crafted descriptors and graph convolutional neural networks that construct a learned molecular representation by operating on the graph ... DOC Chapter 2 Identify the reactants and products in a chemical equation. Write chemical equations using appropriate phase labels, symbols of reaction conditions, and the presence of a catalyst. 2.10 Balancing Chemical Equations. Learning Objectives. Determine if a chemical reaction is balanced. Master techniques for balancing chemical equations. (Example 2.12)
Conventions for Writing Chemical Equations - Arrows, Phases ... This video helps you understand all the letters and numbers you see in a chemical equation. From coefficients and reaction arrows to phases or chemical states, and how to differentiate which...
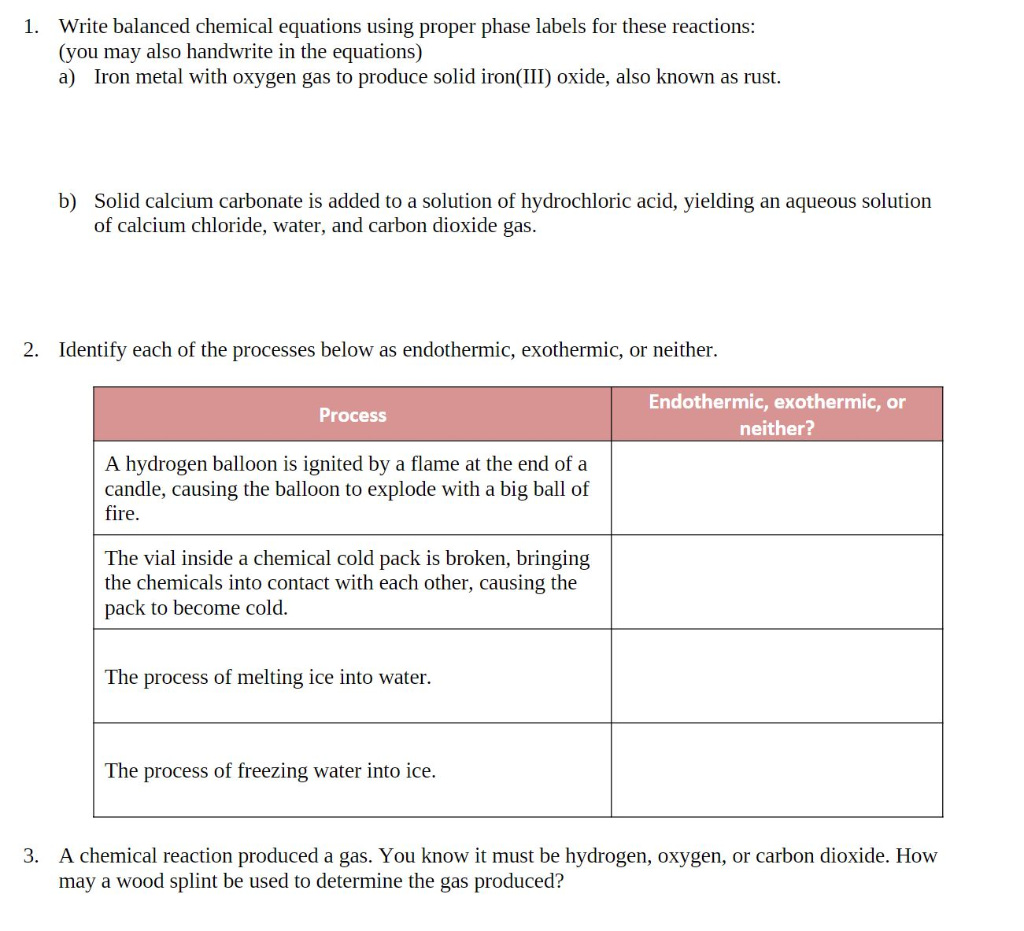
End-of-Chapter Material - Introductory Chemistry- 1st Canadian Edition Chemical equations can also be used to represent physical processes. Write a chemical reaction for the freezing of water, including the proper phase labels. Explain why 4 Na (s) + 2 Cl2(g) → 4 NaCl (s) should not be considered a proper chemical equation. Explain why H2(g) + 1/2 O2(g) → H2O (ℓ) should not be considered a proper chemical equation.
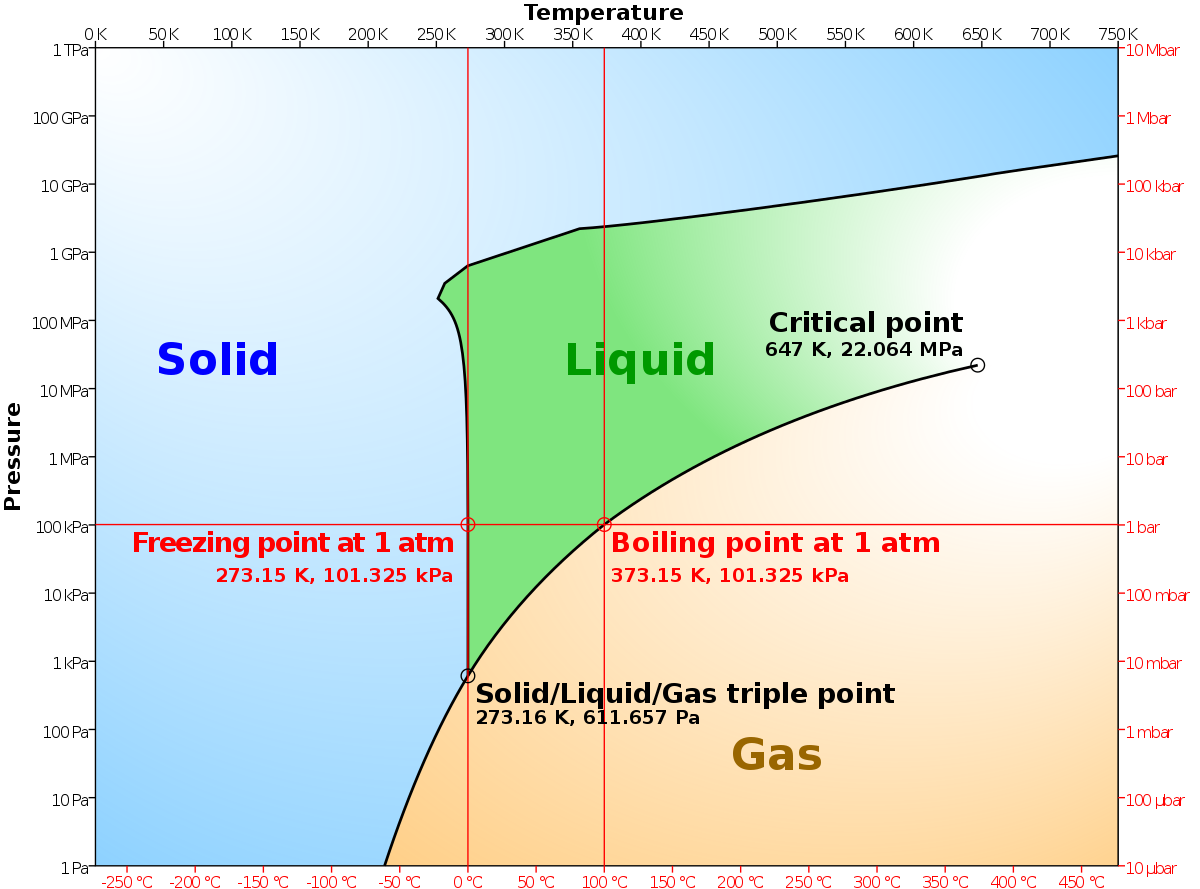
Phase Diagrams - Chemistry - University of Hawaiʻi Using the phase diagram for water given in [link], determine the state of water at the following temperatures and pressures: (a) −10 °C and 50 kPa (b) 25 °C and 90 kPa (c) 50 °C and 40 kPa (d) 80 °C and 5 kPa (e) −10 °C and 0.3 kPa (f) 50 °C and 0.3 kPa Solution
3 Steps for Balancing Chemical Equations - ThoughtCo To do this, you need to be familiar with the properties of various compounds or you need to be told what the phases are for the chemicals in the reaction. Oxides are solids, hydrogen forms a diatomic gas, tin is a solid, and the term ' water vapor ' indicates that water is in the gas phase: SnO 2 (s) + 2 H 2 (g) → Sn (s) + 2 H 2 O (g)
EEP - Electrical Engineering Portal | Energy and Power For All Sep 19, 2022 · LV panels are metal-enclosed switchgear that provides a three-phase power distribution to supply electric power at voltages up to 1000 volts, current up to 10000 amps ...
What are Chemical Equations? Detailed Explanation, Examples - BYJUS An example of an ionic chemical equation is provided below. Chemical Equation: CaCl 2 + 2AgNO 3 → Ca (NO 3) 2 + 2AgCl↓. Ionic Equation: Ca 2+ + 2Cl - + 2Ag + + 2NO 3- → Ca 2+ + 2NO 3- + 2AgCl↓. Comparing the reactants and the products of the ionic equation and the chemical equation, it can be observed that the Ca 2+ ( calcium ion ...
Lagrangian mechanics - Wikipedia Suppose there exists a bead sliding around on a wire, or a swinging simple pendulum, etc.If one tracks each of the massive objects (bead, pendulum bob, etc.) as a particle, calculation of the motion of the particle using Newtonian mechanics would require solving for the time-varying constraint force required to keep the particle in the constrained motion (reaction force exerted by the wire on ...
Regional Screening Levels (RSLs) - What's New | US EPA May 18, 2022 · The labels for adult and child body weight in the Calculator output were corrected. The target risk labels in the Resident Air Supporting Table were corrected. The Inhalation Unit Risk for TCDD was changed to 38 and the Generic Tables were updated. For assistance/questions please use the Regional Screening Levels (RSLs) contact us page. For ...
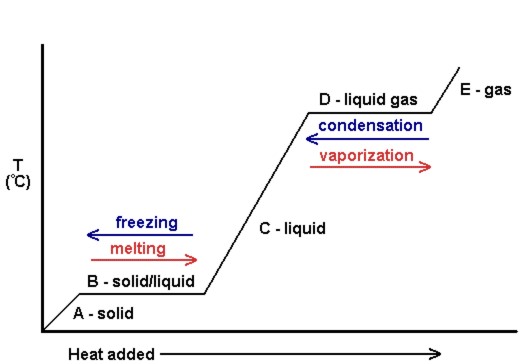
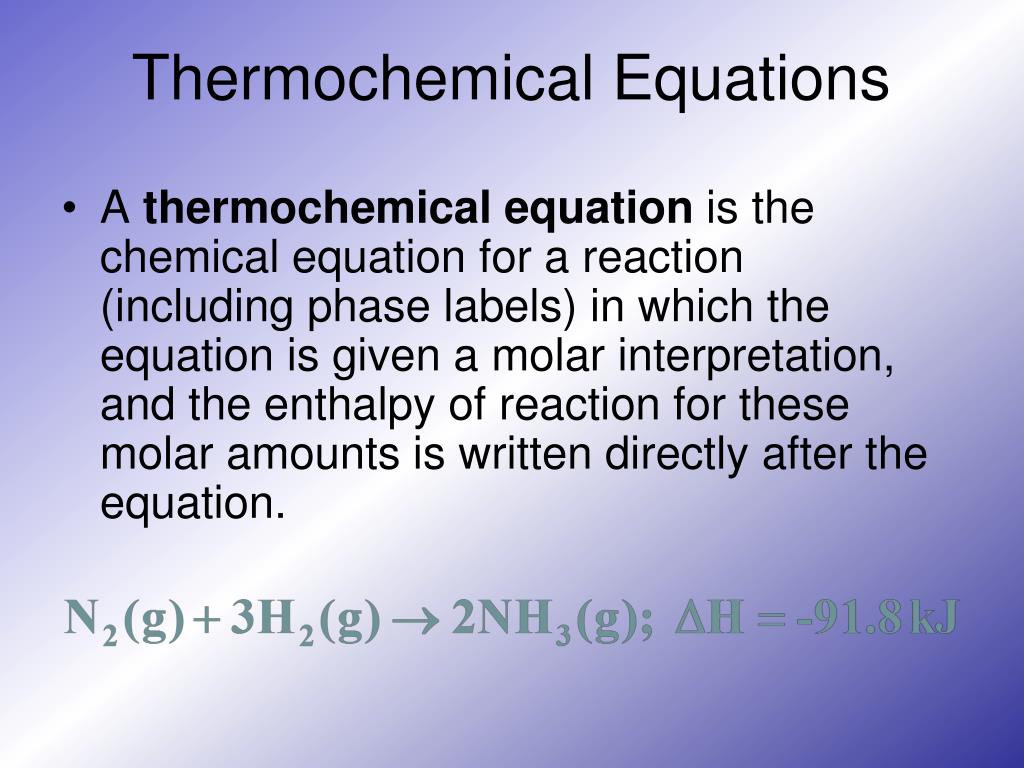
A thermochemical equation is a balanced chemical reaction equation ... An equation which shows both mass and heat relationships between products and reactants is called a thermochemical equation. The following four reactions are examples of thermochemical equations. The first two are exothermic and the last two are endothermic reactions. 2 H 2 (g) + O 2 (g) ----> 2 H 2 O(l) DH = -571.6 kJ
End-of-Chapter Material - Introductory Chemistry - 1st Canadian Edition Chemical equations can also be used to represent physical processes. Write a chemical reaction for the freezing of water, including the proper phase labels. Explain why 4Na (s) + 2Cl 2 (g) → 4NaCl (s) should not be considered a proper chemical equation. Explain why H 2 (g) + ½O 2 (g) → H 2 O (ℓ) should not be considered a proper chemical equation.
Chemical Phase Equilibria and Phase Diagrams | SpringerLink Figure 13.7 shows an example in which there are three phases, two solid solution phases \alpha and \beta and a liquid phase ( l ), at equilibrium at a given temperature, called the eutectic temperature \left ( {T}_ {\text {E}}\right), and a particular composition called the eutectic composition { (x}_\mathrm {B}^ {\text {E}}).
Phase Diagram | Explanation, Definition, Summary & Facts The first curve line (A, T) separated the solid and vapour phase, second curve line (T, B) separates the solid and liquid phase whereas third curve (T, C) separates the liquid and vapour phases of a substance (Fig. 1).
Chapter 5 - Chemical Reactions and Equations - CHE 105/110 ... Many chemical equations also include phase labels for the substances: (s) for solid, (ℓ) for liquid, (g) for gas, and (aq) for aqueous (i.e., dissolved in water). Special conditions, such as temperature, may also be listed above the arrow. For example, 2NaHCO3 (s) − →−200°C Na2CO3 (s) +CO2(g) +H2O(ℓ) Key Takeaways
Phase Rule - Teaching Phase Equilibria Gibbs Phase Rule is expressed by the simple formulation: P + F = C + 2, where P is the number of phases in the system A phase is any physically separable material in the system. Every unique mineral is a phase (including polymorphs); igneous melts, liquids (aqueous solutions), and vapor are also considered unique phases.
Phase diagram - Wikipedia A phase diagram in physical chemistry, engineering, mineralogy, and materials science is a type of chart used to show conditions (pressure, temperature, volume, etc.) at which thermodynamically distinct phases (such as solid, liquid or gaseous states) occur and coexist at equilibrium . Contents 1 Overview 2 Types 2.1 2-dimensional diagrams
End-of-Chapter Material - GitHub Pages Chemical equations can also be used to represent physical processes. Write a chemical reaction for the freezing of water, including the proper phase labels. Explain why 4Na (s) + 2Cl2(g) → 4NaCl (s) should not be considered a proper chemical equation. Explain why H2(g) + 1/2O2(g) → H2O (ℓ) should not be considered a proper chemical equation.
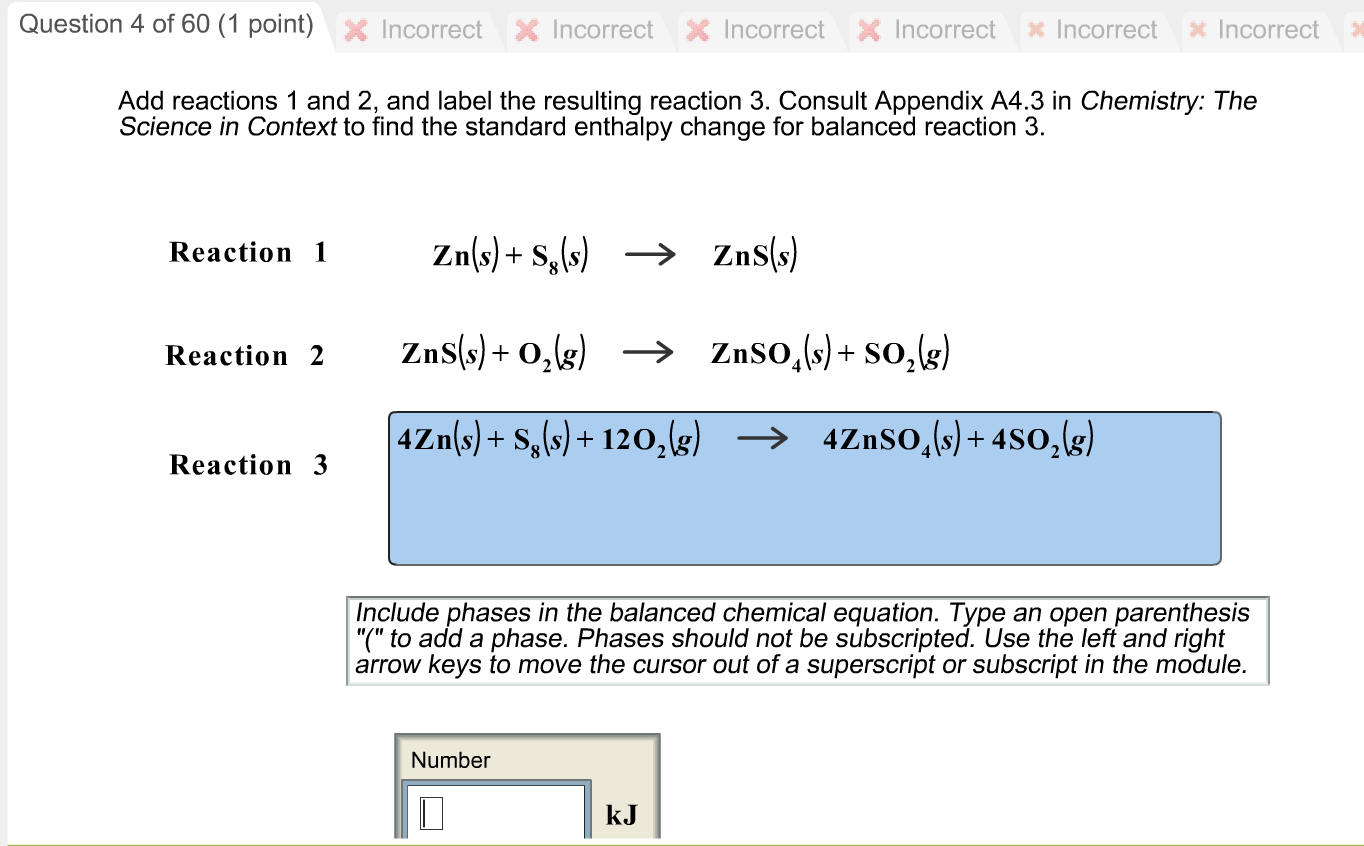
m11-chemical-reactions-lab-report (1).docx - Module 11 Lab... 2. Assuming the reaction was between iron and oxygen in the air and the product contained iron in the +3 oxidation state, write the balanced chemical equation including phase labels for the reaction in Activity 1.
State symbols and phase changes | StudyPug The phase can affect how reactive a substance is, but changing phase (a physical change) is not the same as changing the substance (a chemical change). A fully detailed chemical equation will show the state (or phase) of matter that the atoms or molecules are in. These states are: Solid, given the symbol (s) Liquid, given the symbol (l)
Definition of dissolve and the state label (aq) in chemical equations A colloid is a heterogeneous phase. But above all, do not worry too much about these minor symbolism issues. A chemical equation is not meant to convey everything. It is just a shorthand notation to describe chemical reactions. A real experiment or a research paper should describe the experimental condition with sufficient details in such a way ...
The Chemical Equation - Introductory Chemistry - 1st Canadian Edition Write and balance the chemical equation that represents nitrogen and hydrogen reacting to produce ammonia, NH 3. Answer N 2 + 3H 2 → 2NH 3 Many chemical equations also include phase labels for the substances: (s) for solid, (ℓ) for liquid, (g) for gas, and (aq) for aqueous (i.e., dissolved in water).
Properties of the density functional response kernels and its ... Sep 15, 2022 · When considering the particle-density operator, there are different possibilities, emerging from different possibilities in the labels s and t (and u and v) in the particle-density operator: The labels s and t are associated with the wavefunction of different fragments in the bra and ket, e.g., s with fragment A and t with fragment B ,

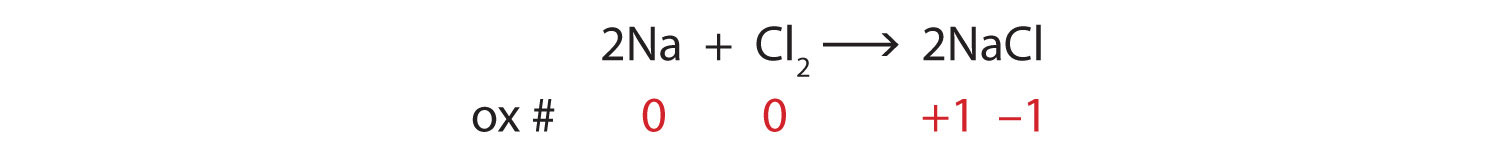











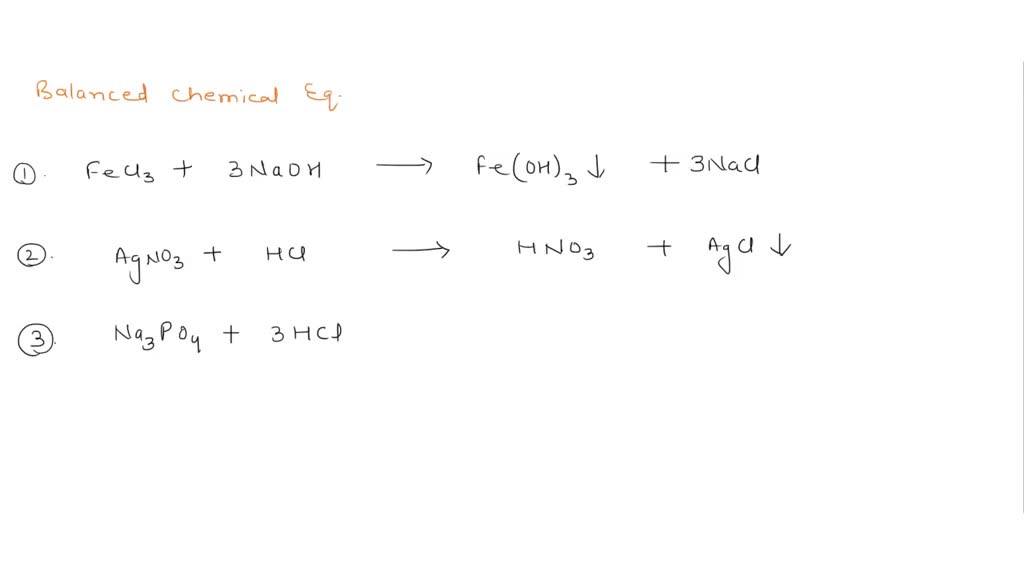





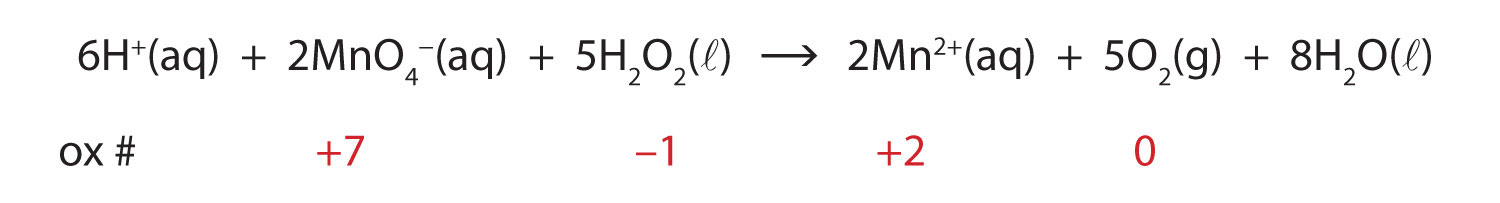


Post a Comment for "38 phase labels in chemical equations"